half life formula for first order reaction
Half-Life of a First-Order Reaction Recall that for a first-order reaction the integrated rate law is given by. T ½ A o 2k For a.

What Is Second Order Of Kinetics Kinetics Ppt Chemistry Classroom Chemistry Basics Physics Lessons
Equations for Half Lives.

. For a first-order reaction the half-life is independent of concentration and constant over time. Where t12 is the half-life in seconds s and k is the rate constant in inverse seconds s-1. The half-life of a second-order reaction is given by the formula 1kR 0.
For a zero-order reaction the half-life equation is given as. Graphical relations and half lives. Derivation of Half-Life Formula for First-Order Reactions latextextlnfracA_tA_0 -ktlatex latextextlnfracA_0A_t ktlatex.
It is a constant and related to the rate constant for the reaction. So our half-life is equal to let me rewrite this here so our half-life t 12 is equal to 693 divided by k where k is our rate constant. The half-life of a reaction is the time required for the reactant concentration to decrease to one-half its initial value.
The first-order reaction half-life equation is given by k 2303 t l o g R 0 R From the definition of the half-life of a first-order reaction at t t12 and R R 02. The half-life of a. The half-life of a second-order reaction is given by the formula 1kR 0.
What is the half-life of a first. K 2303 t l o g R o R In the above expression k is the rate constant t12 is the time R o is. Converting a half life to a rate constant.
Substituting the values in the. T 12 0693k. So here is your half-life for a first order reaction.
Emulsions Where The half. Derivation of the Half-life Formula The rate constant for a first-order reaction is expressed as. Therefore we can set eq A eq equal to eq.
What is the half-life formula for a first order reaction. The half-life of a first-order reaction is given as t 12 0693k. What is the half-life equation for a second-order reaction.
The half-life formula for a reaction depends upon the order of a reaction. First-Order Reactions We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. The half-life of a first-order reaction does not depend upon the concentration of the reactant.
For a zero order reaction A products rate k. Half-life equation for first-order reactions. What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions.
Where The half-life of a reaction is referred to as t 12. A A 0 e k t AA_0 e-kt A A 0 e k t. For a first zero order.
Determining a half life. The half-life of a first-order reaction is given as t 12 0693k. The First Order Half-Life calculator computes the first order half-life based on the temperature dependent rate constant.
We know that at the half-life time eqt_ 12 eq the concentration of the reactant will be half as much as the initial concentration.

Class 12 Chemistry Revision Notes For Chapter 4 Chemical Kinetics Chemical Kinetics Chemistry Notes Chemistry Worksheets

Plastic Antioxidant Plastic Antioxidants Infiltration

We Talk About The Lion S Gate August 8th But Do We Really Understand What It Is First Off It S Hugely Powerful Here S Why Every Year Spirituell Pferde

Ncert Solutions For Class 12 Chemistry Chapter 4 Chemical Kinetics Cbse Tuts Chemicalkineticsclass12ncertsolu Chemical Kinetics Chemistry Order Of Reaction

Pin By Kaiaaa On Gymnastics Gymnastics Quotes Cheerleading Quotes Inspirational Gymnastics Quotes

Reaction Kinetics Chemistry Lessons Chemistry Education Teaching Chemistry

Left Side Acrylic Nails Coffin Pink Long Square Acrylic Nails Acrylic Nails Coffin Short

Ap Chemistry Power Point And Guided Notes Chemical Kinetics Chemical Kinetics Chemistry Lessons Guided Notes

Thermochemical Equations Practice Problems Example 2 Equations Chemistry Problem

Pin By Sammy Dulisse On Combustible Lemons Portal Art Portal 2 Portal Game
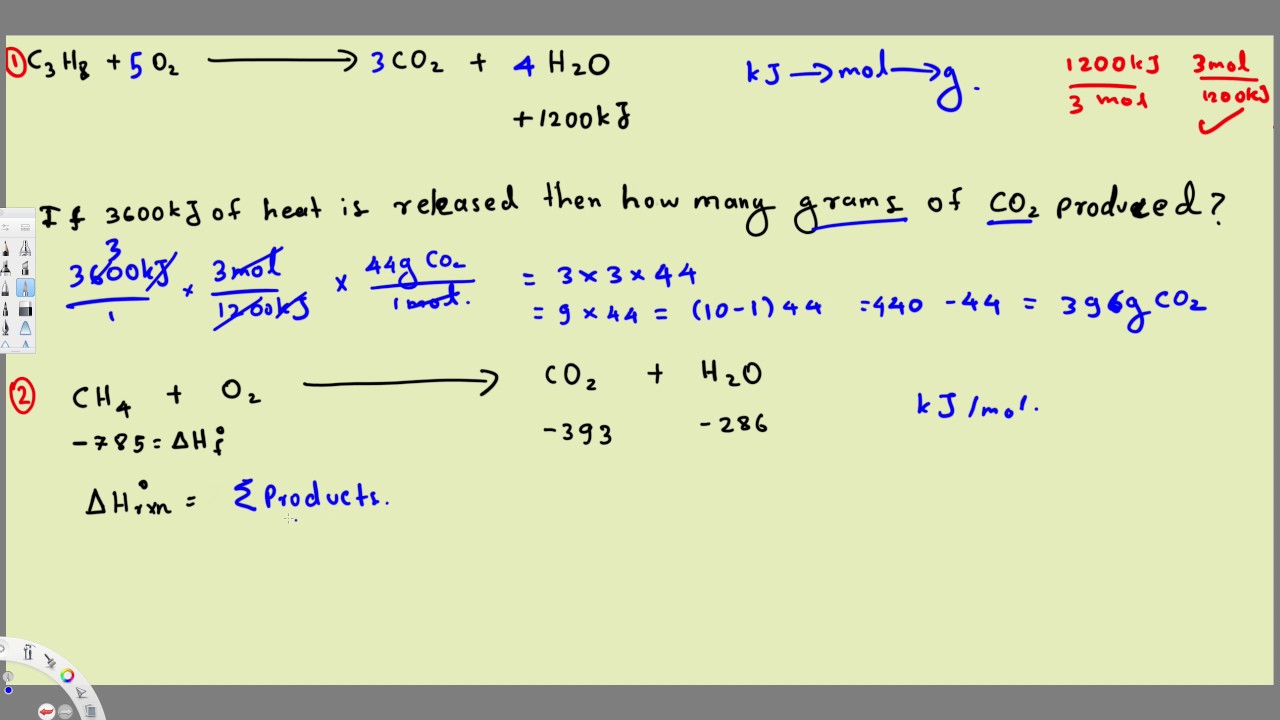
Thermochemistry Equations Formulas Practice Problems Example 2 Equations Chemistry Practice

26 Underrated Apps Every Twentysomething Should Download Right Now Spongebob Of Mice And Men Spongebob Funny

How Do Fireflies Light Up Firefly Insects Beautiful Bugs

Extraction Metals Through Electrolysis 2 Pure Products Industrial Metal

Opposing Reactions Reversible Reactions Types Chemical Kinetics Urdu Hindi Saad Anwar Chemical Kinetics Reaction Types Reactions

Amazing Picture Of Our New Baby Boy Pregnancy Birth And Babies Birth Photos Baby Born Baby Birth

Ncert Solutions For Class 12 Chemistry Chapter 4 Chemical Kinetics Cbse Tuts Chemicalkineticsclass12ncertsoluti Chemical Kinetics Chemistry Chemistry Notes

Electrochemistry Notes Electrochemistry Chemistry Notes Chemistry Lessons
